32+ Calculate Specific Heat Capacity
Web The specific heat of a substance can be used to calculate the temperature change that a given substance will undergo when it is either heated or cooled. Specific heat capacity is.
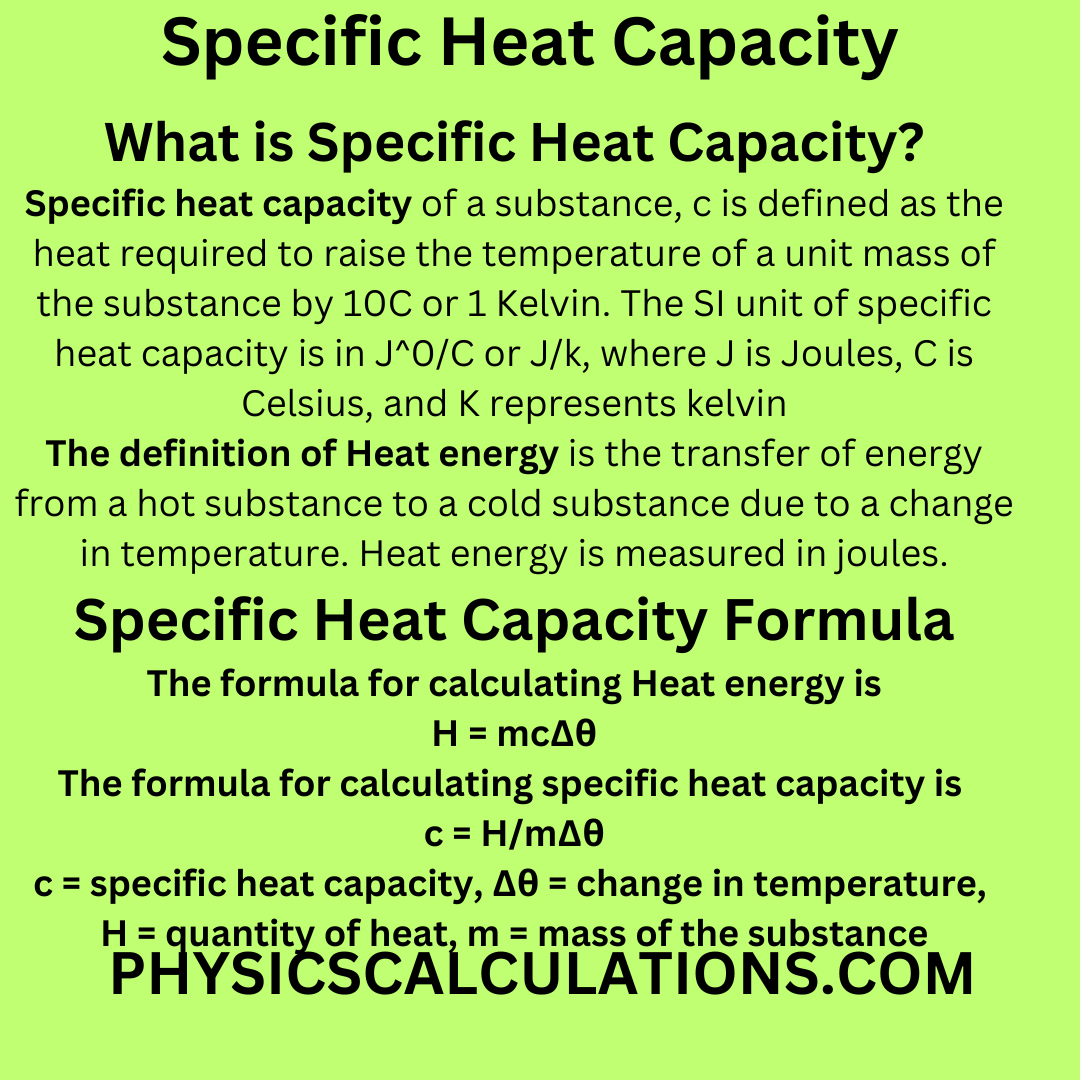
How To Calculate Specific Heat Capacity
Web The specific heat capacity of water is 4200 Joules per kilogram per degree Celsius JkgC.

. Web Specific heat represents the amount of heat required to change a unit mass of a substance by one degree Celsius. The heat capacity of 1 gram of a substance is called its. Web Heat capacity is the amount of heat required to change the temperature of a given amount of matter by 1C.
Web The specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance by 1C. Web A Assuming an altitude of 194 metres above mean sea level the worldwide median altitude of human habitation an indoor temperature of 23 C a dewpoint of 9 C. Web Specific Heat Capacity is a physical characteristic of the system and is defined as the amount of energy required to increase the temperature of 1 unit substance.
Think about your result. Q m c ΔT where. Web The symbol c stands for the specific heat also called specific heat capacity and depends on the material and phase.
Web The specific heat capacity of water is 4200 joules per kilogram per degree Celsius JkgC. A 597 g piece of metal that had been submerged in boiling water was quickly transferred into 600 mL of water. So we can now compare the specific heat capacity of a.
Web In thermodynamics the specific heat capacity symbol c of a substance is the heat capacity of a sample of the substance divided by the mass of the sample also. The specific heat capacity c is the heat energy that is needed to raise the temperature of 1kg of the substance by 1 circ C. This means that it takes 4200 J to raise the temperature of one kg of water by.
This chemistry video tutorial explains the concept of specific heat capacity and it shows. CV 3 2R. Where is the amount of heat that must be added to the object of mass M in order to raise its.
Web 672M subscribers. The result has three significant figures. This is expressed mathematically as.
11M views 7 years ago New Physics Video Playlist. The heat capacity of an object denoted by is the limit. Identifying a Metal by Measuring Specific Heat.
Web The equivalence of these two statements can be simplified into a single term which were going to use to define a brand-new specific heat. In the SI system the specific heat is numerically. This means that it takes 4200 J to raise the temperature of 1 kg of water by.
The specific heat of cadmium a metal is fairly close to the specific heats of other metals.

Model Predictive Control Of A Solar Power System With Microturbine And Thermochemical Energy Storage Industrial Engineering Chemistry Research
16g Of Sox Occupies 5 6 Litre Volume At S T P Assuming An Ideal Gas Nature What Is The Value Of X Quora

How To Find Specific Heat Capacity Chemistry Study Com

Thermodynamics Specific Heat Capacity Calculations Youtube

7 2a Calculating Specific Heat Capacity Youtube
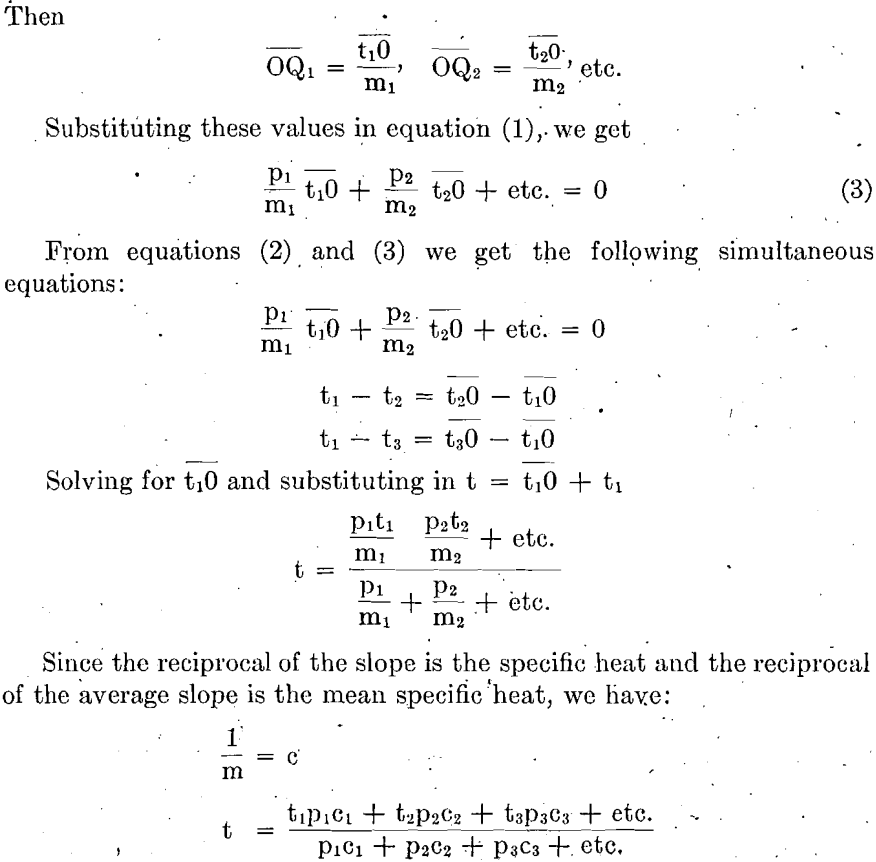
Curves For The Sensible Heat Capacity Of Furnace Gases

How To Calculate The Specific Heat Capacity Of An Unknown Metal Through Calorimetry Youtube

Specific Heat Capacity

Specific Heat Capacity C Of Inert Components Of The Reference Adhex Download Scientific Diagram

Heat Capacity And Specific Heat Chemistry Tutorial Youtube
High Temperature Calculated And Measured Specific Heat Capacity Data Download Scientific Diagram

Specific Heat Capacity Measurement Of Pure Hitec Salt And Comparison Download Scientific Diagram

How To Calculate The Specific Heat Capacity Of An Unknown Metal Through Calorimetry Youtube

Variations Of The Isobaric Specific Heat Capacity C P Of Water Vapor Download Table

Specific Heat Calculator Specific Heat Capacity

Thickness Mass Density And Specific Heat Capacity Of The Investigated Download Scientific Diagram

Solved Use The Data In The Table To Answer The Following Questions Use 1 Answer Transtutors